How did the hydrogen chloride or methanol explosion accident happen?
Hydrogen chloride in methanol is a widely used reagent in organic synthesis, which can be used for purification of amines, esterification of carboxylic acids, etc. It is generally prepared by in situ generation of HCl, such as adding chlorotrimethylsilane (TMSCl) to various alcohols or adding acid chlorides to ethanol, or bubbling gaseous HCl into the alcohol. Because it is a commonly used laboratory reagent, the potential dangers of HCl/methanol have not been brought to the attention of chemists.
Recently, researchers from Oriel Industries of Germany published an article entitled: Safety Case Study. Intrinsic Instability of Concentrated Solutions of Alcoholic Hydrogen Chloride: Potential Hazards Associated with Methanol at the American Chemical Society Org. Process Res. Dev. The article A laboratory safety incident caused by hydrochloric acid/methanol solution was described, aiming to draw the attention of organic synthesizers to such reagents.

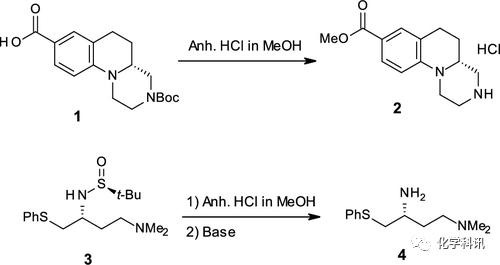
In an antitumor drug synthesis project of Oriel Industries, 4M anhydrous hydrogen chloride/methanol solution is often used to esterify intermediate 1 and form hydrochloride, and remove the sulfinyl group of intermediate 3 The chiral amine 4 is obtained. Because aqueous media are to be avoided, methanolic hydrochloric acid is used.
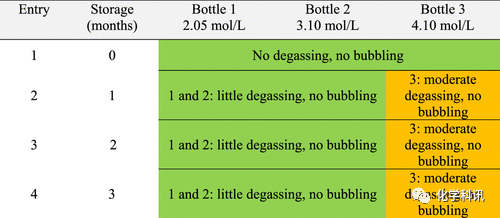
The researchers prepared methanolic hydrochloric acid solutions with concentrations of 2-4 mol/L by bubbling gaseous HCl in MeOH. The first set of 3 bottles was stored at 20–25°C and the second set of 3 bottles at 0–5°C. After storage for 1, 2, and 3 months, one bottle of each group was carefully opened and the concentration titrated.
The stability of methanol hydrochloride was measured by observing the degassing of the liquid in the bottle (degassing, the hissing sound when the cap was opened) and bubbling (bubbling, the bubbles generated after the cap was opened). The results show that the behavior of the solution is related to its concentration, shelf life and storage temperature. Freshly prepared solutions are considered safe to handle (entry 1, Tables 1 and 2). The authors strongly recommend storage at 0–5°C, as the solution after opening the stopper behaves mildly (Table 2). However, proper precautions must be taken when handling solutions stored at 20–25°C, especially 4 mol/L (Table 1, red). In that case, a lot of outgassing and a lot of air bubbles can be observed at any time. The use of 2 mol/L and 3 mol/L solutions can slightly reduce the risk, but substantial accidental outgassing occurs after 1 month of storage (Table 1, entry 2).
Table 1. Behavior of methanolic hydrochloride stored at 20–25°C

Table 2. Behavior of methanolic hydrochloride stored at 0-5°C
At the same time, the authors quantified the HCl concentration of each solution, and the results correlated with the observed behavior (Figure 1). When stored at 20–25°C, the concentration decreases substantially after 1 month, and then decreases slightly after 2 and 3 months. This phenomenon diminishes when the solution is stored at 0–5°C.
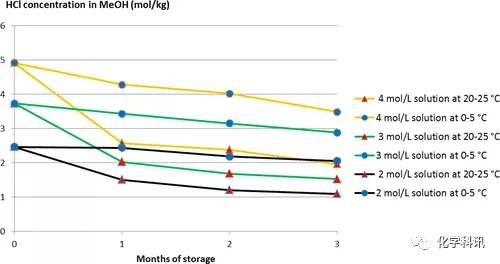
Figure 1. HCl concentration in methanol as a function of time under different conditions
Then, after storage at room temperature for 3 months, qualitative mass spectrometry was performed on the atmosphere in the bottle containing 4 mol/L hydrogen chloride MeOH solution. The main component detected was methyl chloride, with some dimethyl ether. No gaseous HCl was observed. At the same time, water (8% w/w measured by Karl Fisher analysis) was observed from the reaction.
Finally, the authors monitored the methyl chloride and water content of 5–10% w/w hydrogen chloride MeOH solutions stored at 20–25°C (Figure 2). The methyl chloride content in the solution increased within 1 month and decreased in the following months, probably due to evaporation of methyl chloride due to its low boiling point (-23.7°C). After 1 month of storage, the water content increased significantly and then stabilized.
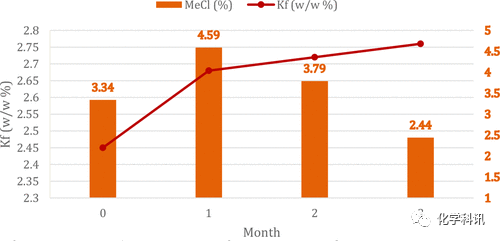
Figure 2. Monitoring of MeCl content over time in 5-10% w/w hydrogen chloride methanol solutions stored at 20°C
To explain why the eruption occurred during pipetting, the authors assumed that the equilibrium of the methanolic hydrochloric acid solution was unstable after several months of storage at room temperature. When the pipette hits the surface of the solution, the methyl chloride dissolved in methanol is suddenly released due to supersaturation, resulting in a spray.
The authors then decided to apply the same approach to ethanol hydrochloride (Tables 3 and 4). In terms of safety, HCL in ethanol is by far the best choice as no degassing or bubbling was observed even at room temperature.
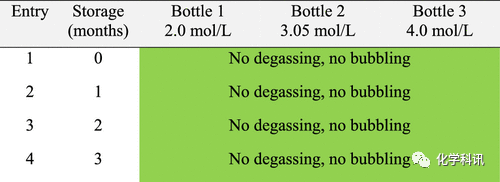
Table 3. Behavior of ethanol/HCl stored at 20–25°C
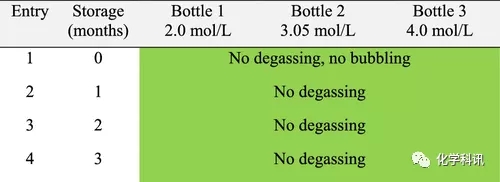
Table 4. Behavior of ethanol/HCl stored at 0–5°C
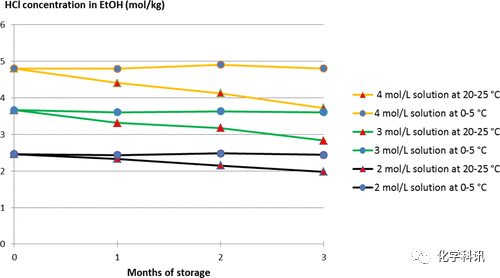
The authors also monitored the HCl concentration over time, and the results are shown in Figure 3. A slight decrease was observed at 20-25°C for the 2 mol/L solution, and the decrease was more pronounced for the more concentrated solution. After 3 months of storage, only about 20% of the HCl was lost, compared to about 60% in methanol. When the solution is stored at 0–5°C, the molarity is very stable with little loss. These results are related to any major events not observed in ethanol.
Figure 3. HCl concentration in ethanol as a function of time under different conditions.
Through this study, the authors provide data on the stability of hydrochloric acid/methanol solutions. After storage, the concentration of HCl will vary depending on time and temperature and the nature of the solvent. If methanol hydrochloride must be used, the authors recommend using this solution immediately after preparation, and for safety reasons, long-term storage of highly concentrated solutions should be avoided. Since no bubbling and degassing events were observed, the authors believe that the HCl/ethanol solution is currently preferred. When this solution was stored at 0-5°C, the change in HCl concentration was negligible.












 关闭返回
关闭返回